Nan Yang
SENIOR MEMBER

- Joined
- May 1, 2010
- Messages
- 5,252
- Reaction score
- 1
- Country
- Location
Chinese scientists in pollution busting 1-in-80 year breakthrough
Zhang Tong in Beijing
Published: 6:00am, 14 Feb, 2023

Researchers from China and Spain say they have developed a more efficient way to remove the compounds that form particulate air pollution from industrial processes. Photo: AFP
Scientists from China and Spain say they have made a rare breakthrough in the hunt for a more affordable and effective way to reduce harmful industrial emissions, using the microporous silicates called zeolites which are at the heart of catalytic converters and other processes to remove contaminants.
and Spain say they have made a rare breakthrough in the hunt for a more affordable and effective way to reduce harmful industrial emissions, using the microporous silicates called zeolites which are at the heart of catalytic converters and other processes to remove contaminants.
The researchers, from the Instituto de Ciencia de Materiales de Madrid (ICMM) and Jilin University’s State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, said they have developed the most porous and stable zeolite so far, called ZEO-3.
The low-cost material’s performance in removing volatile organic compounds (VOCs) – precursors of the most harmful small particles in the environment – is “excellent” compared to other zeolites and metal-organic frameworks, they said.
Li Jian, lead author of the paper published in January by the peer-reviewed journal Science, said the team had achieved something which has eluded scientists in the field of zeolites, which are used in oil refining and fuel cells, as well as biomedicine.
“In the more than 80-year history of human synthesised zeolites, a three-dimensionally stable, ultra-large-porous silicate zeolite has always been the goal of all researchers in this field,” Li said, in an official statement from Jilin University.
“This topic remains a great challenge, with few breakthroughs in the past few decades,” he said.
A diagram showing ZEO-3’s porous structure. Photo: Li Jian
Using pure silica, the researchers were able to design ZEO-3 so that its pores exist along three dimensions, generating a better capacity for large molecules to either stay or react. It was this breakthrough that gives ZEO-3 its performance edge against other zeolites.
Instead of directly building a mesh structure, the team adapted a one-dimensional chain silicate to a three-dimensional strategy, weaving it into a zeolite by using condensation reaction.
In a perspective paper published in the same issue of Science, Russell E. Morris from the University of St Andrews said the mechanism used was similar to “click chemistry”, the methodology for snapping together molecules that won the Nobel Prize in Chemistry last year.
“Silicate chains are aligned to connect to a highly crystalline and almost defect-free product. The new zeolite offers hitherto unforeseen potentials for distinct applications,” Morris said.
The research team said ZEO-3’s structure gives it ultra-high stability, which could have important industrial applications in petrochemicals and many other fields which need to remove the organic molecules from their exhaust gases which contribute to air pollution.
“Until now, zeolites with extra-large pores were not stable, as they were made of germanium instead of silicon,” said ICCM’s Miguel A. Camblor, a co-author of the paper.
Camblor said previous stable zeolites could reach up to seven angstroms. One angstrom is a hundred-millionth of a centimetre. “Now, the new zeolite has a composition of pure silica, and there are pores that reach more than 10 angstroms,” he said.
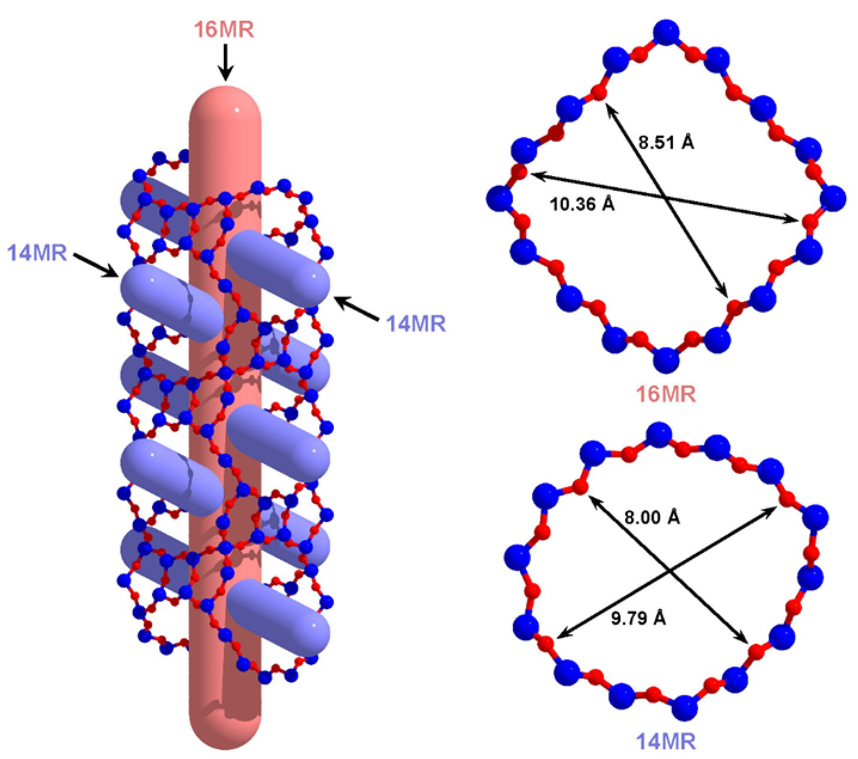
Inside ZEO-3 is a multidimensional, interconnected ultra-large pore system composed of 16 and 14 silicate tetrahedra, respectively, which remains stable even at 1,200 degrees Celsius (648.8 Fahrenheit).
ZEO-3’s density is similar to water, at 1.27 grams per cubic centimetre. But with a specific surface area of 1,000 square metres per gram, the pores in 7g of the material can fill a soccer field when spread out.
- The researchers say they have developed the most stable, effective microporous silicate yet, with implications for industrial emissions
- The key to ZEO-3 stability is its three-dimensional structure of large pores, which has eluded this field of research for eight decades
Zhang Tong in Beijing
Published: 6:00am, 14 Feb, 2023
Researchers from China and Spain say they have developed a more efficient way to remove the compounds that form particulate air pollution from industrial processes. Photo: AFP
Scientists from China
 and Spain say they have made a rare breakthrough in the hunt for a more affordable and effective way to reduce harmful industrial emissions, using the microporous silicates called zeolites which are at the heart of catalytic converters and other processes to remove contaminants.
and Spain say they have made a rare breakthrough in the hunt for a more affordable and effective way to reduce harmful industrial emissions, using the microporous silicates called zeolites which are at the heart of catalytic converters and other processes to remove contaminants.The researchers, from the Instituto de Ciencia de Materiales de Madrid (ICMM) and Jilin University’s State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, said they have developed the most porous and stable zeolite so far, called ZEO-3.
The low-cost material’s performance in removing volatile organic compounds (VOCs) – precursors of the most harmful small particles in the environment – is “excellent” compared to other zeolites and metal-organic frameworks, they said.
Li Jian, lead author of the paper published in January by the peer-reviewed journal Science, said the team had achieved something which has eluded scientists in the field of zeolites, which are used in oil refining and fuel cells, as well as biomedicine.
“In the more than 80-year history of human synthesised zeolites, a three-dimensionally stable, ultra-large-porous silicate zeolite has always been the goal of all researchers in this field,” Li said, in an official statement from Jilin University.
“This topic remains a great challenge, with few breakthroughs in the past few decades,” he said.
A diagram showing ZEO-3’s porous structure. Photo: Li Jian
Using pure silica, the researchers were able to design ZEO-3 so that its pores exist along three dimensions, generating a better capacity for large molecules to either stay or react. It was this breakthrough that gives ZEO-3 its performance edge against other zeolites.
Instead of directly building a mesh structure, the team adapted a one-dimensional chain silicate to a three-dimensional strategy, weaving it into a zeolite by using condensation reaction.
In a perspective paper published in the same issue of Science, Russell E. Morris from the University of St Andrews said the mechanism used was similar to “click chemistry”, the methodology for snapping together molecules that won the Nobel Prize in Chemistry last year.
“Silicate chains are aligned to connect to a highly crystalline and almost defect-free product. The new zeolite offers hitherto unforeseen potentials for distinct applications,” Morris said.
The research team said ZEO-3’s structure gives it ultra-high stability, which could have important industrial applications in petrochemicals and many other fields which need to remove the organic molecules from their exhaust gases which contribute to air pollution.
“Until now, zeolites with extra-large pores were not stable, as they were made of germanium instead of silicon,” said ICCM’s Miguel A. Camblor, a co-author of the paper.
Camblor said previous stable zeolites could reach up to seven angstroms. One angstrom is a hundred-millionth of a centimetre. “Now, the new zeolite has a composition of pure silica, and there are pores that reach more than 10 angstroms,” he said.
Inside ZEO-3 is a multidimensional, interconnected ultra-large pore system composed of 16 and 14 silicate tetrahedra, respectively, which remains stable even at 1,200 degrees Celsius (648.8 Fahrenheit).
ZEO-3’s density is similar to water, at 1.27 grams per cubic centimetre. But with a specific surface area of 1,000 square metres per gram, the pores in 7g of the material can fill a soccer field when spread out.
